What’s Lustromedic?
Lustromedic utilizes Iwasaki Industry Inc.’s expertise in the plastic industry built over long years of developing plastic molding technologies, product quality control and guarantee programs that meet the strict safety and reliability demands of the household goods manufacturing industry in order to contribute to a healthier and happier global society through the creation of previously unprecedented medical equipment.
Iwasaki Industry Inc.’s product policy aims to analyze and resolve all customer complaints in order to develop patient-friendly medical equipment considering usability for medical workers.
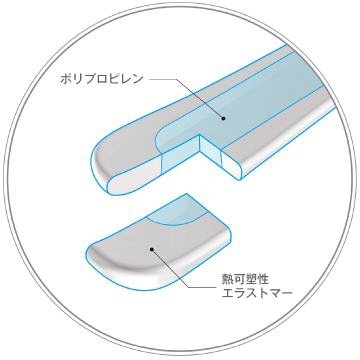

Soft
Not cold
Flexible way to use, Choice your color
We strive to improve the quality of medical equipment through the usage of superior plastic materials.
Medical device manufacturing registration number: 24BZ200037
Type 2 medical device manufacturing and sales license number: 24B2X10011
Type 2 medical device manufacturing and sales license number: 24B2X10011
Copyright@iwasaki-industry. All rights reserved.



